Hingham, MA -- April 15, 2021 -- Applied Tissue Technologies (ATT), an advanced wound care company, announced the publication of a peer reviewed study in Plastic and Reconstructive Surgery Journal® Global Open, "Utilization of a Novel Negative Pressure Platform Wound Dressing on Surgical Incisions: A Case Series".
The study was the first utilizing the PWD's negative pressure wound therapy "NPWT" function in humans. This study completed a Phase II Enhancement of a Small Business Innovation & Research "SBIR" contract awarded originally by the Defense Health Agency as contract number W81XWH-14-C-0015 in 2014. The PWD has successfully completed preclinical testing where it demonstrated strong efficacy when compared to current product offerings in a recent publication and has also demonstrated efficacy with a publication detailing negative pressure at -50mmHg.
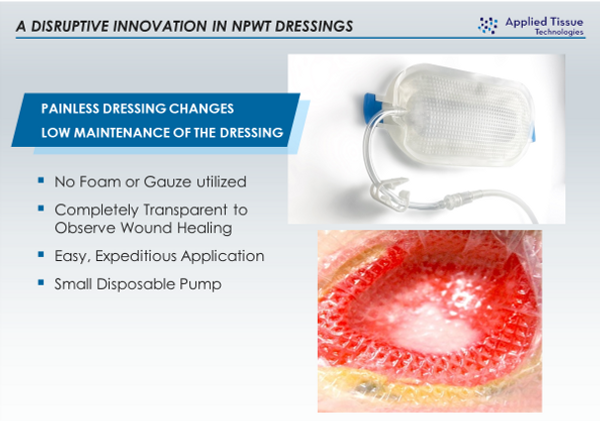
The PWD's NPWT function received it's first patent in 2014 from the USPTO 8,632,523 and 510k clearance from the FDA in 2019. "Negative Pressure Wound Therapy is not a new treatment, but elimination of the problematic components, the fully transparent design and ease of use will make the PWD a compelling choice for wound care practitioners. We are very pleased to see the PWD being used in patients," Michael Broomhead, CEO, ATT.
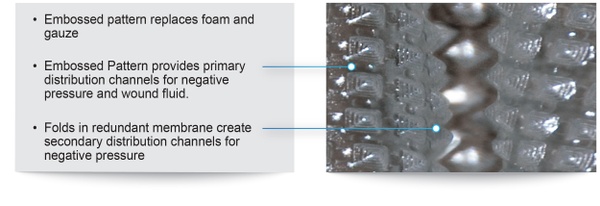
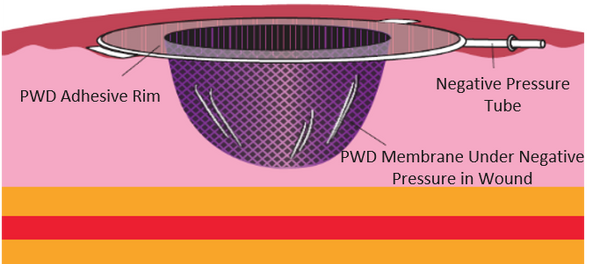
The PWD is a transparent dressing with an integral adhesive base and a permanently embossed impermeable membrane that combines the traditional functions of the NPWT membrane and foam/gauze in currently marketed NPWT devices. In the PWD, no foam or gauze is needed. When the NPWT pump is switched on, the embossed membrane superstructure is redundant which allows it to be pulled into direct contact with all geometries of the wound. The space created between the embossments provides primary channels for air and fluid, while folds in the membrane create secondary channels that provide an even distribution of negative pressure across the wound.
The PWD is a member ATT’s portfolio of advanced wound care products including, the Xpansion® MicroAutografting Kit and establishes a product line of advanced wound care technologies that can reestablish the skin barrier function, deliver topical therapeutics and close wounds with the gold standard of wound healing, an autologous split thickness skin graft.
Applied Tissue Technologies
Applied Tissue Technologies brings advancements in wound healing to patients. This includes research and development supported by the United States Department of Defense to help wounded warriors at the point of injury, instances of prolonged field care and delayed evacuation, and also continuing through advanced levels of military medical care. www.appliedtissue.com @npwtsimplified
The PWD is supported in part by the United States Defense Health Agency (Small Business Innovation Research), USAMRAA, and USAMRMC through contract numbers W81XWH-18-2-0002,
W81XWH-18-C-0143, W81XWH-19-2-0038 and W81XWH-16-0784. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.