Hingham, MA -- January 14, 2018 -- Applied Tissue Technologies (ATT), an advanced wound care company, announced US FDA 510(k) clearance of its Negative Pressure Wound Therapy product, the Platform Wound Dressing (PWD®). Classified by the FDA as a class II device, the patented PWD represents the first-of-its-kind embossed negative pressure wound therapy (NPWT) device to be used without foam or gauze.
“The PWD represents a true innovation in wound healing technology,” said Michael Broomhead, CEO of ATT. “Our company is committed to novel research and developing new technologies in advanced wound care. We believe that the PWD with its embossed NPWT will have a major impact in healing patients’ wounds.”
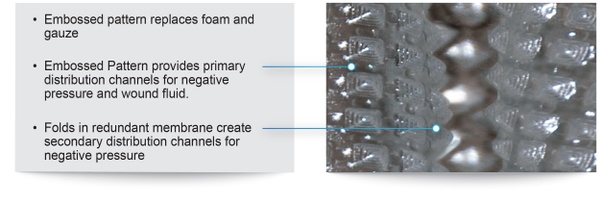
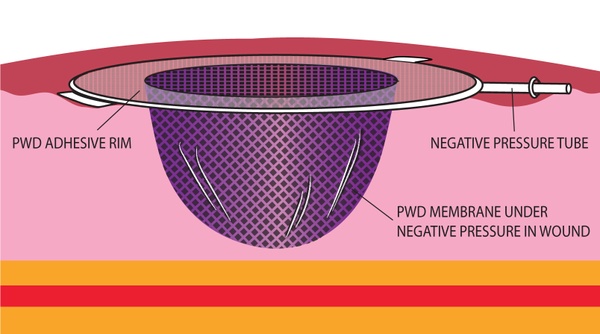
The PWD is a transparent dressing with an integral adhesive base and a permanently embossed impermeable membrane that combines the traditional functions of the NPWT membrane and foam/gauze in currently marketed NPWT devices. In the PWD, no foam or gauze is needed. When the NPWT pump is switched on, the embossed membrane is pulled into direct contact with all geometries of the wound. The space created between the embossments provides primary channels for air and fluid, while folds in the membrane create secondary channels that provide an even distribution of negative pressure across the wound.
Independent of this clearance, ATT has three ongoing clinical trials funded by the United States military to evaluate the PWD.
The PWD adds to ATT’s portfolio of advanced wound care products including, the Xpansion® MicroAutografting Kit and establishes a product line of advanced wound care technologies that can reestablish the skin barrier function, deliver topical therapeutics and close wounds with the gold standard of wound healing, an autologous split thickness skin graft.
Applied Tissue Technologies
Applied Tissue Technologies brings advancements in wound healing to patients. This includes research and development supported by the United States military to help wounded warriors at the point of injury, instances of prolonged field care and delayed evacuation, and also continuing through advanced levels of military medical care. www.appliedtissue.com @npwtsimplified
The PWD is supported in part by the United States Defense Health Agency (Small Business Innovation Research), USAMRAA, through contract numbers W81XWH-18-2-0002, W81XWH-18-C-0143, and W81XWH-16-0784. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
###
Source: Applied Tissue Technologies