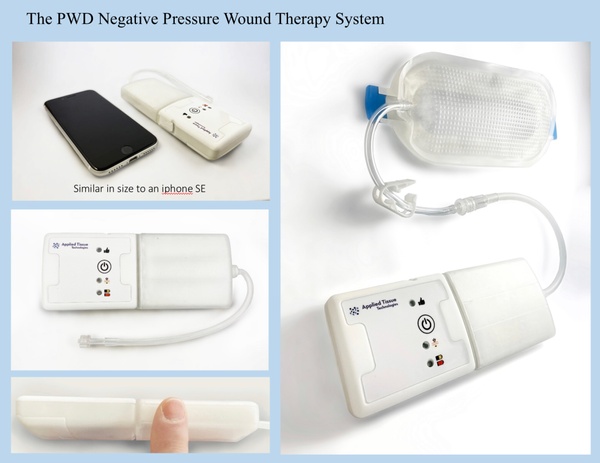
September 23, 2022 -- Boston, MA -- Applied Tissue Technologies (ATT), an advanced wound care company, announced the 510k clearance by the FDA today of its Platform Wound Dressing “PWD®” Negative Pressure Wound Therapy “NPWT” System. This clearance pairs ATT’s revolutionary embossed dressing with a purpose built pump developed from the ground up for use with this dressing.
ATT set out to design a single-use pump that would compliment the simplicity of the transparent embossed dressing. “We wanted an intuitive design when we started out. The pump had to be single-use, compact, powerful and easy to use. Our dressing eliminates the problematic foam associated with NPWT and we were able to achieve a 7 day indication because of that,” commented Michael Broomhead, Applied Tissue’s CEO.
The 7 day indication means Fewer Interventions that will translate to lower costs and most importantly a better patient experience with fewer dressing changes.
The PWD has applications across wound care, from chronic to acute wounds and burns. Recent funding from the Department of Defense has allowed ATT to showcase the PWD’s utility in combat casualty care as a means to halt injury progression in a clinical trial, data will be published later this year. Here the PWD serves as a fixation platform to deliver topical analgesics and antimicrobials and other approved and indicated therapeutics. The device also has applications for civilian treatment of large scale burn/ blast disaster-type injuries.
Recent publications of the PWD’s safety appeared a peer reviewed study in Plastic and Reconstructive Surgery Journal® Global Open, "Utilization of a Novel Negative Pressure Platform Wound Dressing on Surgical Incisions: A Case Series".
The study was the first utilizing the PWD's negative pressure wound therapy "NPWT" function in humans. This study completed a Phase II Enhancement of a Small Business Innovation & Research "SBIR" contract awarded originally by the Defense Health Agency in 2014. The PWD has successfully completed preclinical testing where it demonstrated strong efficacy when compared to current product offerings in a recent publication and has also demonstrated efficacy with a publication detailing negative pressure at -50mmHg.
The PWD is supported in part by the United States Defense Health Agency (Small Business Innovation Research) contract number W81XWH-14-C-0015, USAMRAA, and USAMRMC through contract numbers W81XWH-18-2-0002, W81XWH-18-C-0143, W81XWH-19-2-0038 and W81XWH-16-0784. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.